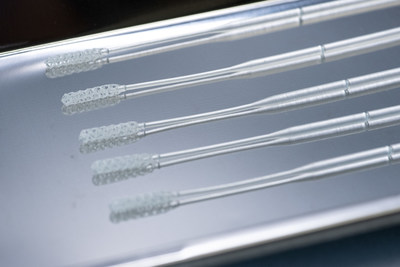
Company Ramps Production of Millions of Test Swabs in the Coming Weeks
SAN FRANCISCO, April 13, 2020 — (PRNewswire) — Origin, the developer of the leading open platform for additive mass manufacturing, is an instrumental additive manufacturing leader collaborating with Beth Israel Deaconess Medical Center (BIDMC) to find a better solution for manufacturing COVID-19 test swabs. Over the last two weeks, Origin has quickly shifted its resources from being a 3D printer manufacturer to become a medical device manufacturer and has started production to address the massive shortage of COVID-19 test kit supply.Health experts have said widespread testing for COVID-19 is the best way to track and trace the spread of the virus, yet America is desperately behind. Test swabs are difficult to manufacture and require design features and qualities that include a mix of stiffness + flexibility, biocompatibility, autoclavability, and specimen capture and carrying efficacy. Traditional test swab production is currently limited to only a couple of factories in the world.
BIDMC, an academic medical center affiliated with Harvard Medical School, selected Origin's 3D-printed test swab to be part of a clinical trial to evaluate it for efficacy and safety. Origin's 3D-printed swab passed a rigorous initial clinical evaluation for human factors, materials testing, and PCR compatibility, and we expect that full results of the clinical trial will be published shortly.
"The nation's need for rapid and widespread testing for COVID-19 has been hampered by a widespread shortage of the swabs needed for testing," said Ramy Arnaout, MD, DPhil, Associate Director of the Clinical Microbiology Laboratories at BIDMC, who has led a multi-disciplinary effort to resolve the shortage and is overseeing the clinical evaluations of 3D-printed swabs at BIDMC. "Innovations in 3D printing hold real promise for our collective efforts to diagnose and treat COVID-19, as well as to flatten the transmission curve."
Origin's test swabs are considered an FDA Class 1 Exempt Device. In addition to BIDMC's clinical testing, Origin's swabs have gone through rigorous testing with the US Army, Origin material partners, universities including UCLA, and independent medical labs.
Origin designed the swabs using the nTop Platform to rapidly iterate many different versions for BIDMC to evaluate, allowing them to quickly hone in on the best design elements for clinical efficacy and patient comfort. Origin worked closely with its Open Material Network to identify the proper medical grade material and to quickly refine optimal processing conditions.
Origin is also part of a new industry consortium called PrintedSwabs.org, which is bringing together the efforts of the 3D printing industry with academia and medical enterprises to supply millions of 3D printed COVID-19 test swabs.
Origin Technology
Origin's technology is a perfect fit for this application because of its remote monitoring and control software, as well as its fine feature lattice capabilities. A single Origin One printer can print up to 1,500 swabs in a single print in under 8 hours. Origin's test swabs are a more reliable and scalable solution than traditional swab manufacturing. Complex, detailed geometries can easily be produced on Origin One and do not require any molds. The test swabs can be produced when needed, anywhere, with only a single material for the supply chain.
"Origin is proud to join together with teams of experts across technology, healthcare and academia to help fast-track efforts to get durable and safe medical devices in the hands of healthcare professionals," said Chris Prucha, co-founder and CEO, Origin. "These are unprecedented times that require unprecedented measures. Given this, Origin has made the decision to focus its efforts solely on developing medical devices and PPE during this crisis. This shift happened quickly and under very difficult conditions, as San Francisco was the first city to order shelter in place. We are pleased to say that the clinical trial results of our test swabs have gone very well, and the positive feedback has enabled us to develop a more comfortable product for patients."
Other Origin COVID-19 Efforts
Origin is working on a number of other PPE projects. The company recently worked on an open source project to produce an ANSI compliant face shield for DLP, SLA printers and SLS printers that is much safer for front line healthcare workers than other FDM face shield designs currently available:
https://github.com/originlabs/origin-opensource-faceshield
Origin also worked with Stanford Engineering on a custom attachment for a full-face snorkeling mask, similar to the N95 mask, that will act as a swap-in-replacement for the snorkel tube: Pneumask: Reusable Full-Face Snorkel Mask PPE Project. The company has also been working with several open source groups as a contributor on product development for other PPE equipment: https://www.gofundme.com/f/MasksOn (include pics of prototypes)
Availability of Origin Test Swabs
Origin's test swabs are available to order now at
https://www.origin.io/npswab
About Origin
Based in San Francisco, CA, Origin is pioneering the concept of Open Additive Manufacturing, a new way to build based on open materials, extensible software, and modular hardware. Origin One, the company's manufacturing-grade 3D printer, uses programmable photopolymerization to precisely control light, heat, and force among other variables to produce parts with exceptional accuracy and consistency. The company works with a network of material partners to develop a wide range of commercial-grade materials for its system, resulting in some of the toughest and most resilient materials in additive manufacturing. The company was founded in 2015 and is led by alumni from Google and Apple. Investors include Floodgate, DCM, Mandra Capital, Haystack, Stanford University, and Joe Montana. Learn more about Origin here:
https://www.origin.io
![]() View original content to download multimedia:
http://www.prnewswire.com/news-releases/origin-3d-printed-covid-19-test-swabs-clinical-trial-and-validation-completed-with-beth-israel-deaconess-medical-center-301039218.html
View original content to download multimedia:
http://www.prnewswire.com/news-releases/origin-3d-printed-covid-19-test-swabs-clinical-trial-and-validation-completed-with-beth-israel-deaconess-medical-center-301039218.html
SOURCE Origin
| Contact: |
| Company Name: Origin
Mary Devincenzi, Steele-Alloy Communications, mary@steele-alloy.com, 408-761-4285 Web: http://www.origin.io |