- FDA's clearance of D2P marks 3D Systems' entry into the Point of Care (POC) market, addresses growing demand for in-house manufacturing
ROCK HILL, S.C., Sept. 12, 2019 — (PRNewswire) — 3D Systems (NYSE: DDD) announced today it received additional 510(k) clearance for its D2P™ (DICOM-to-PRINT) software allowing clinicians to 3D print diagnostic patient-specific anatomic models. 3D Systems is the only company to offer a solution that combines its own software and printers to create 3D printed patient-specific anatomic models for diagnostic purposes in an unmatched breadth of medical specialties including: cardiovascular, craniofacial, gastrointestinal, genitourinary, neurological, and musculoskeletal applications. D2P relies on unique automatic segmentation tools driven by deep learning that allow medical practitioners to quickly create accurate, digital 3D anatomic models from medical imaging data. With the additional FDA clearance, D2P addresses the growing demand by point of care (POC) institutions for in-house manufacturing using an accurate and reliable 3D segmentation solution that can produce 3D printed models.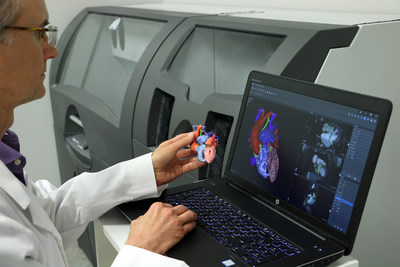
D2P now also includes the latest advancements in deep learning image processing technology and virtual reality visualization allowing hospitals and device manufacturers to significantly reduce the time associated with the creation of 3D models.
The software also includes a volumetric VR solution enabling instant views of patient scans in a 3D environment - facilitating surgical planning and conversations between medical staff and their patients.
"We are used to going into surgery with uncertainties and an arsenal of contingency plans," said Dr. Solomon Dadia, deputy director of the orthopedic-oncology department and director of the 3D surgical center at Souraski Medical Center in Tel-Aviv. "With 3D printed models and enhanced 3D visualization tools such as VR, we are able to gain a better understanding of the surgery and pathology we are going to treat. This allows us to come up with a more precise surgery plan designed to deliver a better surgical outcome."
In accordance with the FDA announcement on new guidelines for 3D printed patient-specific anatomic models in 2017, diagnostic quality models must be an output of a Class II regulated medical device software. 3D Systems is the only company to offer both a software solution and compatible printers of its own that meet this regulatory requirement. Anatomic models can be produced using a variety of 3D Systems printing technologies - ColorJet Printing, MultiJet Printing, Stereolithography, and Selective Laser Sintering – including materials that are capable of sterility and biocompatibility.
"The capabilities offered in D2P give the healthcare professional an unprecedented toolset for deeper medical understanding across most medical specialties," said Ran Bronstein, vice president, chief research and operation officer, 3D Systems. "Our proprietary advanced visualization technology is changing how medical imaging data is used in a variety of formats such as virtual reality and 3D printed physical models."
To learn more about 3D Systems' solutions, please visit the 3D Systems website.
Forward-Looking Statements
Certain statements made in this release that are not statements of historical or current facts are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the company to be materially different from historical results or from any future results or projections expressed or implied by such forward-looking statements. In many cases, forward-looking statements can be identified by terms such as "believes," "belief," "expects," "may," "will," "estimates," "intends," "anticipates" or "plans" or the negative of these terms or other comparable terminology. Forward-looking statements are based upon management's beliefs, assumptions, and current expectations and may include comments as to the company's beliefs and expectations as to future events and trends affecting its business and are necessarily subject to uncertainties, many of which are outside the control of the company. The factors described under the headings "Forward-Looking Statements" and "Risk Factors" in the company's periodic filings with the Securities and Exchange Commission, as well as other factors, could cause actual results to differ materially from those reflected or predicted in forward-looking statements. Although management believes that the expectations reflected in the forward-looking statements are reasonable, forward-looking statements are not, and should not be relied upon as a guarantee of future performance or results, nor will they necessarily prove to be accurate indications of the times at which such performance or results will be achieved. The forward-looking statements included are made only as of the date of the statement. 3D Systems undertakes no obligation to update or review any forward-looking statements made by management or on its behalf, whether as a result of future developments, subsequent events or circumstances or otherwise.
About 3D Systems
More than 30 years ago, 3D Systems brought the innovation of 3D printing to the manufacturing industry. Today, as the leading AM solutions company, it empowers manufacturers to create products and business models never before possible through transformed workflows. This is achieved with the Company's best-of-breed digital manufacturing ecosystem - comprised of plastic and metal 3D printers, print materials, on-demand manufacturing services and a portfolio of end-to-end manufacturing software. Each solution is powered by the expertise of the company's application engineers who collaborate with customers to transform manufacturing environments. 3D Systems' solutions address a variety of advanced applications for prototyping through production in markets such as aerospace, automotive, medical, dental and consumer goods. More information on the company is available at
www.3dsystems.com.

![]() View original content to download multimedia:
http://www.prnewswire.com/news-releases/3d-systems-draws-on-healthcare-expertise-to-deliver-fda-cleared-d2p--industrys-only-company-to-create-patient-specific-diagnostic-anatomic-models-using-its-own-software-and-printers-300917207.html
View original content to download multimedia:
http://www.prnewswire.com/news-releases/3d-systems-draws-on-healthcare-expertise-to-deliver-fda-cleared-d2p--industrys-only-company-to-create-patient-specific-diagnostic-anatomic-models-using-its-own-software-and-printers-300917207.html
SOURCE 3D Systems
| Contact: |
| Company Name: 3D Systems
Investor Contact: Email: Email Contact Media Contact: Nicole York, Email: Email Contact Web: http://www.3dsystems.com Financial data for 3D Systems |